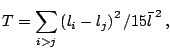 |
(3.1) |
Quantitative measurement of local structural order in the computational models of liquid water (and other associated liquids) is an intrinsically difficult problem. Conventional structure descriptors, such as radial distribution functions cannot be used to adequately evaluate structure in the specific regions of complex model systems like multicomponent solutions or solutions of large biological molecules (Vaisman and Berkowitz, 1992). Another set of structural descriptors, the geometric and thermodynamic parameters of hydrogen bond network depend on arbitrary values in the hydrogen bond definition (Vaisman et al., 1994). Statistical geometry enables a robust and accurate approach to addressing the problem of structure characterization in water. The snapshot conformations from the molecular simulation of water or aqueous solution by molecular dynamics, Monte Carlo, or other method can be easily tessellated and geometrical parameters of the resulting Delaunay simplices can be measured. Tetrahedrality is a quantitative measure of the degree of distortion of the Delaunay simplices from the regular tetrahedra, that was introduced by Medvedev and Naberukhin (1987) for simple liquids, but can be easily extended to water and other systems. Tetrahedrality is calculated as:
The distribution of water tetrahedrality in different layers around solutes depend on the nature of the solute. In the case of charged ions, like ammonium, the difference between tetrahedrality of bulk water and the ammonium hydration water is particularly strong due to the strong hydrogen bonding between water and solute. The peak of the distribution of the tetrahedrality of the ammonium hydration water is shifted to the right which indicates that the hydration water is less tetrahedral than bulk water (Fig. 3.2). Conversely, water in the first hydration shell of methane is just slightly more tetrahedral than the bulk water. Thus, the hydration water around ammonium is significantly more distorted than that around methane as one could expect in the case of hydrophilic and hydrophobic hydration, respectively (Vaisman et al., 1994).
![\includegraphics[width=\textwidth,clip]{text/4-3/fig2.eps}](img8147.gif)
|
It is worth to note that the presence of hydrophobic solute changes the distribution of water tetrahedrality in the same way as the decrease of temperature. This observation is consistent with the concept of the decrease of 'structural temperature' of water, surrounding hydrophobic particles, that has been discussed in the literature for a long time. The decrease in the structural temperature corresponds to the increased structural order of water, because any structural characteristic of liquid must be a monotonically decreasing function of temperature. Distribution of tetrahedrality entirely complies with this requirement.
The influence of both solutes on the water tetrahedrality is almost unobservable beyond the first hydration shell. The distribution of tetrahedrality for both solutions is similar at both cutoff radii (Vaisman et al., 1994). This result indicates that the distribution of tetrahedrality is not sensitive to the treatment of long-range interactions. Distribution of tetrahedrality beyond the first hydration shell is similar to that in pure water.